Vaccination Study
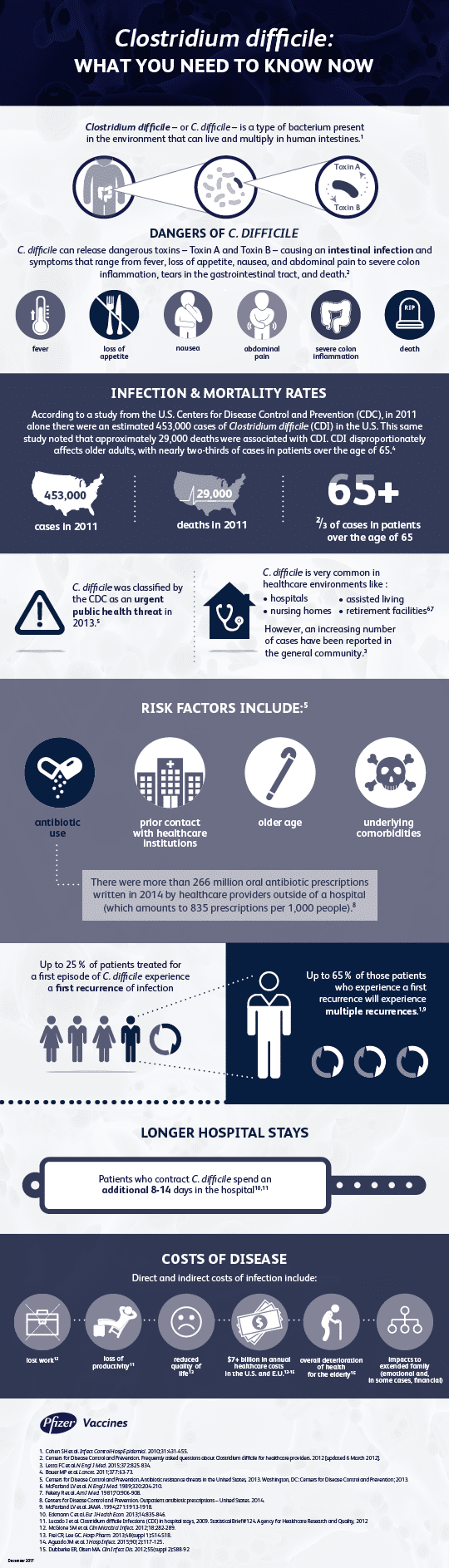
Clostridium difficile (C. diff)






Dr. Aaron N. Hartman, Dr. Raymond Decker, and Lind Reiss, CFNP are evaluating a potential prevention for Clostridium difficile.
C. difficile (C. diff) is one of the most common causes of healthcare-related infections. A clinical research study is currently evaluating an investigational vaccine for its safety and effectiveness in reducing the chance of getting C. diff.
Aaron Hartman, MD
Principal Investigator
Raymond Decker, MD
Sub-Investigator
Lind Reis, CFNP
Sub-Investigator
What is Clostridium difficile?
C. difficile (C. diff) is one of the most common causes of healthcare-related infections.
C. diff is a type of bacteria that lives in the human intestines and its spores can survive outside of the body for months. These spores are resistant to cleaning and are most commonly transmitted in healthcare settings. People can become infected by touching surfaces that are contaminated or by coming into contact with a healthcare worker that has the bacteria or spores on their hands.
People most at risk of getting sick due to C. diff are those who are taking antibiotics, being treated in a healthcare facility, the elderly and people who are already ill. This infection can cause symptoms that include frequent diarrhea, inflammation of the colon, fever, nausea and abdominal pain; in severe cases it can be life-threatening.
The Clover Study is evaluating an investigational vaccine that may help decrease your chance of getting sick due to C. diff.
Why Should I Participate?
Benefits
The purpose of the Clover Trial is to evaluate the safety and efficacy of an investigational vaccine in reducing the chances of getting sick due to C. diff. The knowledge gained from this study may help others in the future.
Risks
The clinical research team will discuss all study risks with you and answer any additional questions you may have.
How to Participate
If you are interested in participating, you will go through a series of screening assessments to
determine your eligibility to get started please call (804) 893–CARE, that is 893-2273. Or, fill out the form below and we will follow up with you.
If you qualify and choose to participate, you will be randomly assigned to receive the investigational vaccine or a placebo, like the flip of a coin.
Eligible participants will receive study related tests and procedures at no cost.
Am I Eligible?
You may be eligible to participate in this clinical study if you are 60 years old or older and are able to say yes to at least one item below:
- Have received oral or injectable antibiotics within the last 12 weeks, or
- Have had at least 2 visits to the emergency room, or
- Had a hospitalization of at least 2 nights in the last 12 months, or
- Have a planned hospitalization that will require a stay of 2 or more nights, or
- Are a resident in a nursing facility, or
- Have had at least 10 outpatient visits in the last 12 months
Note: These are not the only eligibility criteria for this clinical research study, and other criteria may exclude you. A clinical research team member will help determine if you meet all necessary criteria to participate.
About the Clover Trial
Your participation in this study is expected to last between 18 and 42 months and will require 5 office visits within the first 7 months. You will also receive periodic contact from the study team, including regular electronic reminders of your participation and routine telephone calls after 12 months and at the end of the study. Approximately 16,000 patients are expected to participate in the study globally. During the study, you will be given an electronic diary and asked to record any symptoms you might be having. If you have diarrhea 3 or more times within 24 hours, you will be asked to collect a sample of the diarrhea. We will provide a kit for this and arrange for a courier to collect the sample.
You may also have the following assessments during the study period: physical exam, vital signs, urine tests and stool sample(s). All participants will be asked to provide blood samples.
You will be randomly assigned (like the flip of a coin) to receive either the investigational study vaccine or a placebo. This product will be given to you by injection. The placebo looks like the study vaccine, but has no active ingredient in it. You will not know if you are receiving the active vaccine or the placebo.
Interested In Discussing this Trial?
Call us at (804) 893–2273
Or, please complete the form below so we can follow up with you.